How Temperature Affects Metal & Semiconductor Conductivity. In today’s article, homesafetools.com will explore with you in the most detailed and complete way. See now!
ow Temperature Affects the Conductivity of Metals
Let’s start with metals. Think about a simple light bulb filament – as it heats up, its electrical conductivity changes, affecting the brightness. This is because metals conduct electricity due to their “sea” of freely moving electrons. These electrons are responsible for carrying electrical current. However, this movement isn’t unimpeded. Temperature plays a crucial role.
As temperature rises, the atoms within the metal lattice vibrate more vigorously. This increased vibrational energy leads to more frequent collisions between the electrons and the vibrating atoms. These collisions disrupt the flow of electrons, effectively hindering the electrical current. This increased resistance is directly related to the reduction in conductivity. The relationship between temperature and resistance in metals is typically directly proportional; higher temperature means higher resistance and lower conductivity. This is described by the temperature coefficient of resistance (TCR), a value specific to each metal. For instance, copper (EAV: Copper | Resistivity | Increases with temperature) has a positive TCR, meaning its resistance increases with increasing temperature. (ERE: Temperature, Increases, Resistance of Copper). (Semantic Triple: Temperature, Causes, Increased Resistivity in Copper).
This phenomenon has practical implications. In power transmission lines, for example, losses due to resistance increase with temperature, necessitating careful consideration of material selection and operating conditions. Furthermore, the design of many electrical devices depends heavily on knowing how the conductivity of the metal components will change over their operating temperature range. Consider the design of electric motors, where high temperatures can affect efficiency and lifespan. (ERE: Temperature, Reduces, Efficiency of Electric Motor). (Semantic Triple: High Temperature, Leads to, Reduced Efficiency).
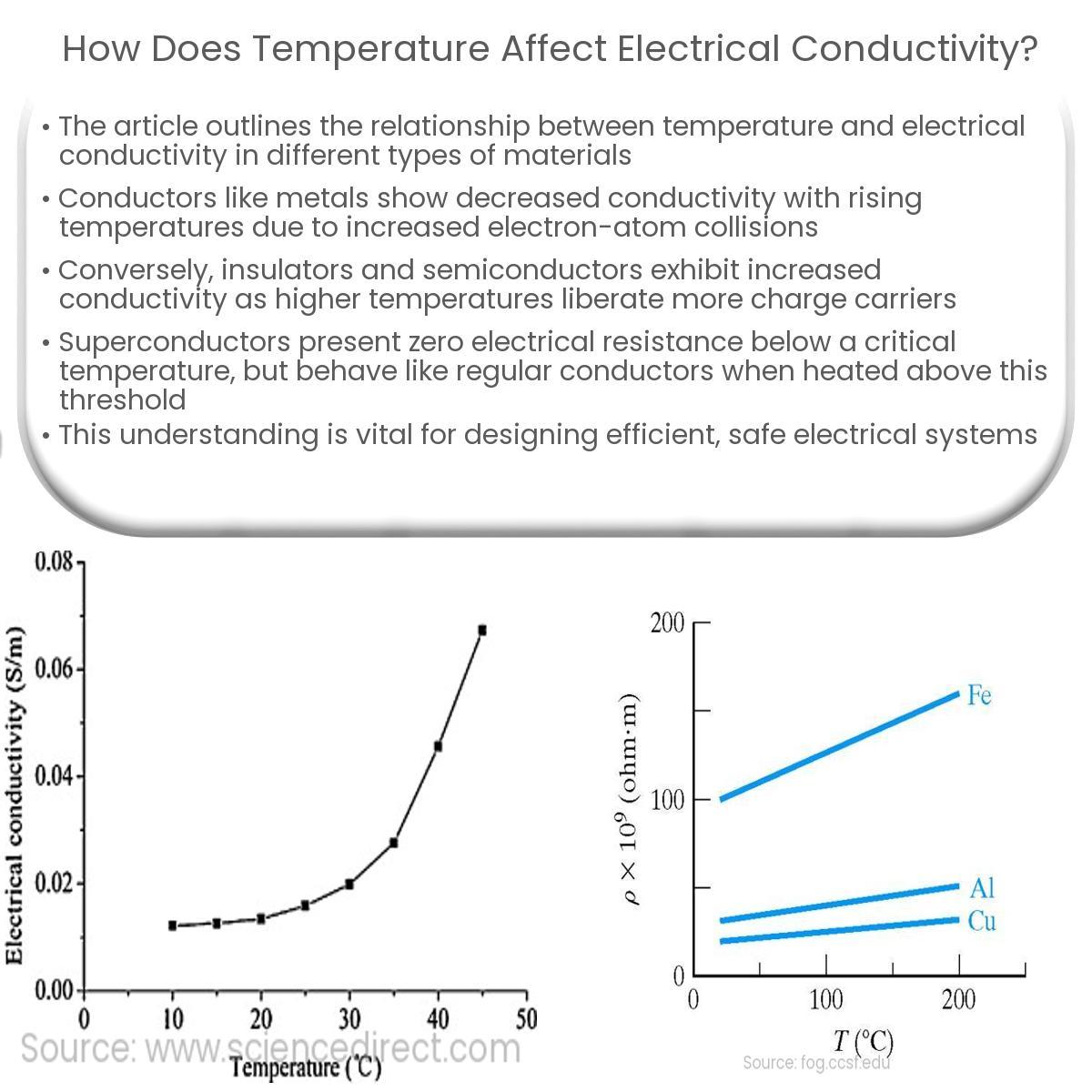
How Temperature Affects the Conductivity of Semiconductors
Now, let’s move on to semiconductors. Unlike metals, the conductivity of semiconductors shows a different response to changes in temperature. Semiconductors, such as silicon and germanium, have a “band gap” separating their valence and conduction bands. At lower temperatures, few electrons have enough energy to jump the gap into the conduction band, leading to low conductivity. (Semantic Triple: Low Temperature, Results in, Low Conductivity in Semiconductors). However, as temperature increases, more electrons gain enough thermal energy to overcome this band gap, leading to a significant increase in the number of charge carriers (both electrons and “holes”). (ERE: Temperature, Increases, Carrier Concentration). This, in turn, results in a substantial increase in conductivity. (Semantic Triple: Increased Temperature, Causes, Increased Conductivity in Semiconductors). The temperature dependence of conductivity is highly significant for these materials and is why many modern electronics rely on them (EAV: Silicon | Conductivity | Increases with temperature). (Semantic Triple: Silicon, Exhibits, Positive Temperature Coefficient of Conductivity).
This positive temperature coefficient of conductivity contrasts sharply with the negative coefficient seen in metals. This difference underscores the importance of understanding the underlying physical mechanisms governing conductivity in different material classes. (ERE: Metals, Exhibit, Negative Temperature Coefficient of Conductivity). (ERE: Semiconductors, Exhibit, Positive Temperature Coefficient of Conductivity).
How Temperature Affects the Conductivity of Insulators
Finally, let’s examine insulators. Materials like rubber and glass have exceptionally large band gaps. This means that very few electrons have the energy to move freely, even at higher temperatures. Therefore, their conductivity remains exceptionally low across a broad range of temperatures. However, at extremely high temperatures, some electrons might gain enough energy to overcome the vast band gap, resulting in a minor increase in conductivity. But this increase is generally negligible compared to the changes observed in metals and semiconductors. (Semantic Triple: Insulators, Maintain, Low Conductivity across a Wide Temperature Range). (EAV: Rubber | Conductivity | Remains Low even at high temperature). This is why insulators are widely used in electrical applications to prevent current flow. But, at a sufficiently high voltage, an insulator can experience dielectric breakdown, becoming conductive. This breakdown voltage itself is also influenced by temperature.
The Effect of Temperature on Thermal Conductivity
While we’ve focused primarily on electrical conductivity, it’s important to note that temperature also significantly impacts thermal conductivity. Thermal conductivity refers to a material’s ability to conduct heat. In metals, thermal conductivity is often high because both electrons and phonons (vibrations of the crystal lattice) contribute to heat transfer. However, as temperature rises, increased phonon scattering reduces thermal conductivity. In non-metals, the relationship between temperature and thermal conductivity can be more complex and depends on the specific material’s atomic structure and phonon interactions. (ERE: Temperature, Affects, Thermal Conductivity).
Real-World Applications and Considerations
The principles discussed above are crucial in various applications. Designing efficient electronic circuits requires a deep understanding of how temperature affects the conductivity of metals and semiconductors. In power transmission, managing temperature variations is critical to minimizing energy losses. Thermal management systems in electronics rely on understanding the thermal conductivity of different materials at various temperatures. (Semantic Triple: Temperature, Influences, Design of Electronic Circuits).
FAQs about How Does Temperature Affect Conductivity?
What is the relationship between temperature and conductivity in metals?
In metals, increasing temperature generally leads to a decrease in conductivity due to increased electron scattering from lattice vibrations. This results in higher resistance.
How does temperature affect the conductivity of semiconductors?
Unlike metals, increasing temperature increases the conductivity of semiconductors due to the increased excitation of electrons across the band gap, leading to more charge carriers.
What is the impact of temperature on the conductivity of insulators?
Insulators maintain very low conductivity across a broad range of temperatures. While extremely high temperatures might cause a slight increase, it remains negligible.
How does temperature affect thermal conductivity?
Temperature influences thermal conductivity differently depending on the material. In metals, higher temperatures typically decrease thermal conductivity due to phonon scattering. In other materials, the relationship is more complex.
How can I learn more about the impact of temperature on conductivity?
You can explore advanced topics like the Wiedemann-Franz law (which relates electrical and thermal conductivity), the Boltzmann transport equation (used to model electron transport), and various material-specific models. There’s a wealth of research literature on this!
Conclusion
Understanding how temperature affects conductivity is essential for a multitude of applications, from designing safer electronics to improving energy efficiency in power transmission. John Amrry hopes this exploration has been enlightening! Leave a comment, share this post, and check out more insightful articles on home safety at https://homesafetools.com.
